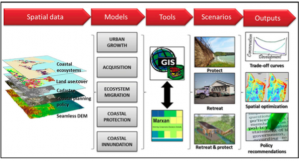
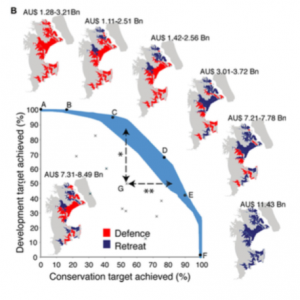
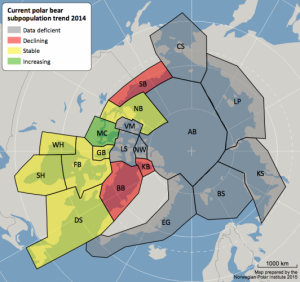
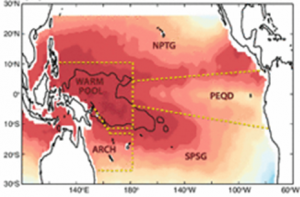
Global Warming is Creating an All-Female Sea Turtle Population
By: Konnor Payne, SRC Intern
Unbeknownst to the majority of people, sea turtles have an attribute, like many reptiles, in which the sex of the animal is determined by temperature. This characteristic is called “temperature-dependent sex determination”, which means the sex, of a sea turtle, is determined during the second trimester of incubation. Eggs at 27.7°C or below will become male sea turtles and eggs at 31°C or greater will become female sea turtles. Between these two temperatures (27.7°C and 31°C), the sex, of the sea turtle, is random (Yntema and Mrosovsky, 1982). As a consequence of this basic reproductive component, coupled with global warming increasing the temperature of the nest, a bias has formed in the sex ratios of all sea turtles towards the females (Laloë, 2016). All around the world are reports of 100% female clutches found in critical nesting sites in Barbados, Caribbean, Cyprus and the Mediterranean. The feminization of the sea turtle population has resulted in 85.9-93.5% of all sea turtles developing as females (Laloë, 2016). Projections predict that females will make up over 95% of the hawksbill population by 2045 and 2028 for leatherbacks, but green sea turtles passed this percentage in 2009 (Laloë, 2016).

Figure 1. Sea turtle eggs in an underground nest on a beach.
Although the sex is heavily skewed towards females the mating behaviors of sea turtles has prevented population issues from arising currently. Female sea turtles are not monogamous and will mate with multiple partners during a breeding season allowing fewer males necessary to fertilize all clutches (Pearce & Avise, 2001). Females can store sperm in their bodies for extended periods of time to fertilize multiple clutches at the appropriate time, reducing the frequency in which females need to contact males (Lee, 2008). The two sexes have separate breeding seasons in which the males breed more often than the females (Hays et al., 2014). These combined mating behaviors help to alleviate the problems of a biased sex population, but with the newest IPCC report indicating only increasing future global temperatures, the males will become too rare to replenish the population.

Figure 2. Female green sea turtles on a beach in Maui preparing to make a nest to lay their eggs.
Not only is there a sex bias, but global sand temperatures have begun to rise above the optimal temperature range and into the lethal zone above 32.4°C, depleting the already low populations (Moran et al., 1999). All seven species of sea turtles are classified as critically endangered, endangered or vulnerable by the International Union for the Conservation of Nature. The sand surrounding the clutch of eggs is warmed by the Sun and the atmosphere, as well as the metabolic activity of the eggs (Ackerman et al., 1985). At higher temperatures the metabolic activity of the hatchlings increases, such that the oxygen levels in the nest can decline to suffocate the hatchlings (Ackerman et al., 1985). If a hatchling does not suffocate the heat may cause thermal inhibition of muscle movement preventing them from leaving the nest (Moran et al., 1999). In the past hatchling rates were consistently above 90%, but projections show that by 2100 hatching success will gradually drop to 50.95-78.92% (Laloë, 2017). The heat from the global warming will cause causalities of sea turtles that are already struggling, which will emphasize the female dominance even more.
The future appears grim for sea turtles but there are strategies to help balance the sex ratio and lower heat stress on the hatchlings. The common problem of hatchling death and skewed sex ratio is the nest has too high of a temperature so the methods to solve both problems are to reduce the temperature of the surrounding sand. Sprinkling water at night or over a shaded area of sand consistently lowered the temperature of the sand near the nest by about 2.25°C (Jourdan, 2015). However, the source of water has to be underground via pipes or another method as an above ground source is heated in the day and causes even higher fluctuations in sand temperature than without sprinkling. The area has to be shaded by a tarp or natural cover as moist sand uncovered will increase in temperature in the day more than without water added. The temperature of the sand decreases the deeper the nest is made, as a nest one meter lower than another is about 1°C cooler (Jourdan, 2015). Once nests are identified they can be dug up and moved deeper into the sand as the hatchlings will still be able to climb out of the nest since larger female sea turtles typically dig deeper nests than the average and have similar emergence rates (Jourdan, 2015). Most sea turtles nest during the warmest months of the year and evidence suggests that they can adapt to the warming temperatures to alter their nesting times to cooler months (Hays, 2014). Although humans have accelerated global warming, that now threatens the existence of all sea turtles, there are methods in which they can be saved.
Work Cited:
Ackerman RA, Seagrave RC, Dmi’el R, Ar A (1985) Water and heat exchange between parchment-shelled reptile eggs and their surroundings. Copeia 1985:703–711
Hays, G. C., Mazaris, A. D., & Schofield, G. (2014). Different male vs. female breeding periodicities help mitigate offspring sex ratios skews in sea turtles. Frontiers in Marine. Science, 1, 43.
Jourdan, J., and M. M. P. B. Fuentes. “Effectiveness of strategies at reducing sand temperature to mitigate potential impacts from changes in environmental temperature on sea turtle reproductive output.” Mitigation and adaptation strategies for global change 20.1 (2015): 121-133.
Juskova, Isabella. “Unsplash.com.” Unsplash.com.
Laloë, Jacques-Olivier, et al. “Climate change and temperature-linked hatchling mortality at a globally important sea turtle nesting site.” Global change biology 23.11 (2017): 4922-4931.
Laloë, Jacques-Olivier, et al. “Sand temperatures for nesting sea turtles in the Caribbean: Implications for hatchling sex ratios in the face of climate change.” Journal of Experimental Marine Biology and Ecology 474 (2016): 92-99.
Lee, P. L. M. (2008). Molecular ecology of marine turtles: new approaches and future directions. Journal of Experimental Marine Biol- ogy and Ecology, 356, 25–42.
Moran KL, Bjorndal KA, Bolten AB (1999) Effects of the thermal environment on the temporal pattern of emergence of hatchling loggerhead turtles Caretta caretta. Mar Ecol Prog Ser 189:251– 261
Pearce, D. E. & Avise, J. C. (2001). Turtle mating systems: behavior, sperm storage, and genetic paternity. Jounral of Heredity, 92, 206-211.
Yntema CL, Mrosovsky N (1982) Critical periods and pivotal temperatures for sexual differentiation in loggerhead sea turtles.
Yong, Mohamed. “Sea Turtle Egg.” Wikipedia.org, 7 Sept. 2007.