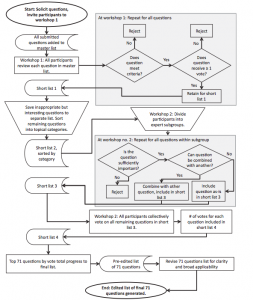
How the complexity of the average marine organism life cycle affects MPA efficiency
By Elana Rusnak, SRC masters student
Marine Protected Areas, or MPAs, are the global “National Park System” of the ocean. There are a variety of protection levels, ranging from multi-use zones where certain activities may only be restricted seasonally, to no take-zones where only non-extractive activities are permitted (i.e. SCUBA diving and mooring a boat), and no-use zones, where there are no activities permitted (Science2action.org, 2015). They are designed to protect a geographic area whose boundaries encompass everything from the surface of the ocean to the ocean floor, and all organisms that live within its borders. MPAs are theoretically designed to protect ecosystem structure, function, and integrity, enhance non-consumptive opportunities, improve fisheries, and expand knowledge and understanding of marine systems (Stoner et al., 2012). These jurisdictions are often put in place to protect the habitat of a certain target species, but yield an additional benefit wherein all of the other organisms that live in that species’ habitat are also protected, as long as they stay within the bounds. For example, a study by Bond et al. in 2017 showed that the establishment of a marine reserve in Belize helped a Caribbean Reef Shark population go from declining (caused by overfishing), to stable over the course of roughly 10 years.
In terrestrial environments, National Parks/Reserves often encompass the entire geographic distribution of a target species. For example, Yellowstone National Park was used in 1995 to reintroduce the Gray Wolf (Canis lupis) back into the wild, after being hunted nearly to extinction (Philips & Smith, 1997). They generally live their entire lives within the park, and as such, their population grew to a sustainable level, and they were subsequently taken off the US Endangered Species List in 2008 (USFWS, 2008).
A Map of the various wolf packs within Yellowstone National park, by Yellowstone_wolfmap.jpg: work of a National Park Service employeederivative work: Rrburke (talk) – Yellowstone_wolfmap.jpg, Public Domain, https://commons.wikimedia.org/w/index.php?curid=7770275
Unfortunately, success stories of this magnitude are not often seen in the marine environment for a few different reasons: MPAs are more difficult to enforce, as they are in a 3D environment where depth is a factor. This is amplified by the fact that many MPAs are created in countries without the resources to maintain them properly (Bennet & Dearden, 2014). Moreover, the ocean is an ever-changing environment, with water flow and fluid dynamics having major effects on every ecosystem. Additionally, a key factor that influences MPA efficacy is the unique life cycle of most marine organisms. A fundamental difference between organisms on land, and in the ocean, is that marine organisms have larval stages. Most fish and other marine organisms (corals, invertebrates, etc.) do not give live birth, or hatch their eggs in a stable environment like land animals. Instead, many reproduce by spawning, which is releasing their millions of eggs and sperm into the water column in hopes they will connect with each other. Once the eggs are fertilized and the larvae begin developing, they are subject to the forces of nature, and move wherever the current takes them. They are also more or less microscopic at this point, and are often a food source for larger fish. Because of this, the larvae of a tuna or blue marlin could be eaten by the very fish that they themselves prey on. This unique circular pattern, coupled with the fact that larval dispersal can span hundreds of miles in the ocean, makes completely protecting a species in a single MPA very challenging (Cowen et al., 2006).

Queen Conch (Lobatus gigas) by Daniel Neal from Sacramento, CA, US – CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=41777013
According to a study by Cowen et al. in 2006, larval dispersion of coral is affected by many factors, including how long the larvae stay in the water column before attaching to the substrate, directed horizontal/vertical movement of the larvae in the water column, and the adult spawning strategies themselves. All of these put together result in larval dispersal distances of anywhere from 10-100km. This dispersal is also seen in the endangered Queen Conch (Lobatus gigas) in the Bahamas. The Exuma Cays Land and Sea Park is an MPA in the Bahamas, and there is a conch population inside the park that has been shown to be slowly dying of old age. This can be attributed to the fact that larvae are not making it into the park because the population that would be supplying them with larvae is outside of the protected area and is being overfished (Stoner et al, 2012; Kough et al. 2017). The MPA does not cover the entire geographic distribution of the conch, and therefore, it can be seen that this life-cycle complexity is affecting the efficacy of this protected area. There have been proposals to create MPA-networks that would protect multiple populations, which may increase larval recruitment (larvae reaching an area and settling down there) and consequently, target species survival. All of this is evidence that shows we need to approach protecting terrestrial and marine species from different angles, since ecosystem type is clearly not the only fundamental difference between them.
References
Bennett, N. J., & Dearden, P. (2014). Why local people do not support conservation: community perceptions of marine protected area livelihood impacts, governance and management in Thailand. Marine Policy, 44, 107-116.
Bond, M. E., Valentin-Albanese, J., Babcock, E. A., Abercrombie, D., Lamb, N. F., Miranda, A., … & Chapman, D. D. (2017). Abundance and size structure of a reef shark population within a marine reserve has remained stable for more than a decade. Marine Ecology Progress Series, 576, 1-10.
Cowen, R. K., Paris, C. B., & Srinivasan, A. (2006). Scaling of connectivity in marine populations. Science, 311(5760), 522-527.
Kough, A. S., Cronin, H., Skubel, R., Belak, C. A., & Stoner, A. W. (2017). Efficacy of an established marine protected area at sustaining a queen conch Lobatus gigas population during three decades of monitoring. Marine Ecology Progress Series, 573, 177-189.
Philips, M. K., Smith, D. W. (1997). Yellowstone Wolf Project – Biennial Report (1995-1996). National Park service.
Science2action.org, 2015. Marine Managed Areas: What, Why, and Where. Science to Action.
Stoner, A. W., Davis, M. H., & Booker, C. J. (2012). Abundance and population structure of queen conch inside and outside a marine protected area: repeat surveys show significant declines. Marine Ecology Progress Series, 460, 101-114.
United States Fish and Wildlife Service, 2008. “Species Profile – Gray Wolf. https://www.fws.gov/home/wolfrecovery/