Ocean Acidification and Your Dinner: Impacts of Marine Seafood
By Emily Rose Nelson
RJD Intern
Ocean acidification is a term commonly used in the world of marine science. This process can most easily be described as the lowering of oceanic pH due to increased atmospheric carbon dioxide concentrations. However, there is much more to this complicated process, which could mean great changes for the future of our oceans.
It is no surprise that atmospheric carbon dioxide levels are increasing. Since the industrial revolution atmospheric CO2 concentrations have increased from 280 ppm to 396 ppm, and this number is expected to increase to 800 ppm by the year 2100. Unfortunately, much of this excess CO2 finds its way to the ocean, changing the natural chemistry of the water. When atmospheric CO2 is added to seawater a series of chemical reactions naturally occurs. The addition of excess CO2 shifts the equilibrium of this reaction series, resulting in increased hydrogen and lower carbonate concentrations. Because pH is equal to –log [H+] increased H+ concentrations have lowered the pH from pre-industrial 8.2 to a 7.8 (projected 2100). Carbonate is essential for calcifying organisms such as mussels, shrimp, some coral species, and more. Carbonate ions combine with calcium ions naturally found in seawater to form CaCO3, the skeletal material for many organisms. Lowered carbonate concentrations make it more difficult to form this compound, thus more difficult to calcify, and in some cases survive.
In recent years a number of experiments have been done looking at the effects of ocean acidification on fishes at current and near future CO2 levels. Overall, this research has concluded that ocean acidification effects brain functioning in fishes. Fish displayed bolder and more risky behavior when exposed to higher CO2 concentrations. For example, a test done on clown fish showed that at near future CO2 levels (850 (µatm) all fish were attracted to predators. Conversely, at CO2 levels of 390 µatm they avoided predators. Sigh and hearing are also impacted. Under normal conditions, Damselfish displayed nervous, anti-predatory, behaviors when shown a possible predator through an aquarium. Given the same situation and higher CO2 concentrations theses behaviors were much less apparent. In addition, there was a great deal of failed lateralization. Fish have a distinct tendency to turn either right or left and this tendency was lost at higher concentrations. These impacts of ocean acidification have been demonstrated on a number of tropical reef species. Experiments have not yet been conducted on commercially valued fish, however it is likely that many of the same responses will result.
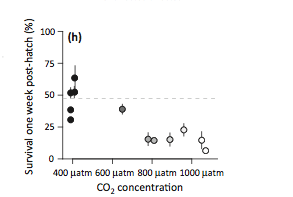
One of many recent studies has found significantly decreased survival at higher CO2 concentrations one week after hatching of Menida beryllina, a fish species commonly found in estuaries. (Branch et al. 2012)
The cause of this change in brain functioning has been traced to the neurotransmitter y-aminobutyric (GABA)-A. With increased CO2 concentrations (GABA)-A is excited. When larval clownfish were treated with a chemical, gabazine, (GABA)-A excitement ceased. This rapidly reversed the changes in brain functioning. Because (GABA)-A is found in a wide variety of species it can be concluded that increased CO2 concentrations will have widely seen implications.
Fished invertebrates show varied responses across taxa. Overall, shelled molluscs are effected the most. With increasing carbon dioxide levels it becomes increasingly difficult for calcification to occur; the shell becomes thinner and weaker. Other bivalves, including bay scallops, hard clams, and eastern oysters, show decreased survival, development, and growth. On another note, ocean acidification shows little to no change to both cephalopods and crustaceans. Juvenile cuttlefish added 22-55% more CaCO3 ot their internal cuttle bones at extreme levels (4,000-6,000 (µatm). Studies done on spider crabs, Antarctic krill, and European lobster leave the organisms relatively unharmed with increased CO2. Unlike most crustaceans, adult shrimp survival decreased in acidified water.
If CO2 levels continue to increase as expected it is likely that we will have to adjust what seafood we eat. Models of the North Pacific, west coast of the USA, and Australia all predict that ocean acidification will reduce total fish catches. Research on tropical fish has predicted a loss of sensory ability that is predicted to threaten many commercial fish. If these predictions hold true it is likely that total fish consumption will have to be reduced. Decreased carbonate concentrations will lead to less mussels and oysters, leaving humans to eat mainly crabs, lobsters, shrimp, and squid. Because most of the predictions were created using lab experiments there is still a great deal of uncertainty regarding how entire marine food webs will be impacted by ocean acidification. There is no current method capable of stopping this problem; the best option is to continue efforts to reduce carbon dioxide emissions.
REFERENCES
Branch, Trevor A., Bonnie M. DeJoseph, Liza J. Ray, and Cherie A. Wagner. “Impacts of Ocean Acidification on Marine Seafood.” Trends in Ecology and Evolution(2012).


Leave a Reply
Want to join the discussion?Feel free to contribute!