by James Keegan, RJD intern
Global demand for renewable energy is increasing as nations strive to decrease their carbon emissions, pollution, and dependence on non-renewable resources like coal or oil. Because of its energy output and potential to compete in energy markets, wind energy may be the most promising renewable energy (IEA 2013). Consequently, wind energy has been rapidly expanding, reaching a capacity in 2012 that was 5 times greater than the capacity in 2005 (IEA 2013). Expectations for offshore wind energy are particularly high, as wind conditions are often stronger and more stable over sea (Bergström et al. 2014). Moreover, offshore wind farms allow for higher total levels of energy production and larger windmill units, which may be transported and constructed more easily (Bergström et al. 2014). Offshore wind farms also appease dissenters who contest that the visual disturbance caused by windmills ruins the aesthetics of local landscapes. As technology has advanced, the average capacity of turbines and size of offshore wind farms have been increasing, and they are being installed in deeper waters further from the coast (Bailey et al. 2014). By the end of 2013, operational wind farms were in an average water depth of 16 meters (~52 ft.) and 29 kilometers (~18 miles) from shore in Europe (Bailey et al. 2014). With such an increase in the encroachment on the marine environment, the focus on offshore wind farms’ impact on the local marine ecosystem continues to grow as well.

Windmill park in Oresund between Copenhagen, Denmark and Malmo, Sweden. (Photo source: Wikimedia Commons)
Although conservationists are studying the impact of offshore wind farms, the novelty of the technology and construction processes make it difficult to identify and quantify all of the stressors. The first commercial scale offshore wind farm, Horns Rev 1, only became operational in 2002 (Bailey et al. 2014). Moreover, the impact offshore wind farms have depends on local ecosystems, which can vary wildly. However, the understanding of the potential effects of offshore wind farms on marine ecosystems, as well as marine biodiversity, is steadily improving as empirical evidence from operational wind farms accumulates (Bergström et al. 2014). The two phases of offshore wind farms, construction and operation, have different potential effects, and these effects differ among species, depending on their likelihood of interaction with the structures and cables, sensitivities, and avoidance responses (Bailey et al. 2014). The major environmental concerns related to offshore wind farms are increased noise levels, risk of collisions, changes to benthic and pelagic habitats, alterations to food webs, pollution from increased vessel traffic, bird collisions, electromagnetic fields, and release of contaminants from seabed sediments (Bailey et al. 2014). However, offshore windmill farms also benefit the local ecosystem by acting as artificial reefs as well as de-facto marine reserves, providing protection against fishing pressures. With both beneficial and adverse contributions to the marine environment, offshore wind farms continue to intrigue conservationists.
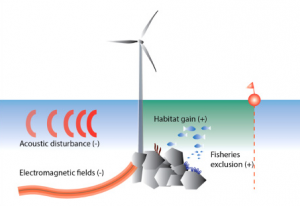
Figure 2: An overview of the main pressures from offshore wind farms during the operational phase. Expected effect on the local abundance of marine organisms is indicated as (+) aggregation/increase, or (−) avoidance/decrease (Bergström et al. 2014).
The two fundamental stages of the development and use of offshore wind farms provide their own challenges and have their own environmental concerns. The construction phase is likely to have the greatest impact on marine mammals, and the activities of greatest concern are pile driving and increased vessel traffic (Bailey et al. 2014). Pile driving is the mechanical process of imbedding poles into sediment to provide foundational support for the proposed structure. Pile driving is currently the most common method used to secure turbine foundations to the seafloor, and it emits a considerable amount of noise (Bailey et al. 2014). The sounds emitted during pile driving could potentially cause hearing damage, mask calls, or displace animals as they leave the area to avoid the noise (Bailey et al. 2014). Moreover, the noise produced by pile driving can cause mortality and tissue damage in fish (Bergström et al. 2014). Alternatively, wind farms can use gravity foundations, which anchor windmills essentially by means of their own weight. The intensity of the noise caused by their installation is low, and animals are likely to return soon after the exposure has ended, thus lowering the impact (Bergström et al. 2014). However, this type of installation does cause high sediment dispersal due to dredging, or the removal of underwater sediments, but typically organisms inhabiting the sites of gravity foundation wind farms are tolerant of turbidity (Bergström et al. 2014). In addition to the installation of the windmills themselves, the increased vessel traffic associated with surveying and installation activities also creates the risk of collision and noise disturbance to marine mammals, sea turtles, and fish (Bailey et al. 2014). Although the construction phase of offshore wind farms only creates problems for the marine environment, its impact can be curtailed by avoiding significant habitats and seasons.
The operational phase of offshore wind farms requires a more challenging and complex assessment because of the duality of its environmental impacts. Like the construction phase, the operational phase raises concerns about acoustic disturbances, but from electricity generation and boat traffic for service and maintenance. The acoustic disturbances caused by the operation of the windmills are within the hearing range of fish and mammals, but underwater sound levels are unlikely to reach dangerous levels or mask acoustic communication of marine mammals (Bergström et al. 2014), (Bailey et al. 2014). Transmission cables transporting the generated electricity produce electromagnetic fields, which can affect cartilaginous fish, like sharks, which use electromagnetic signals in detecting prey (Bergström et al. 2014). The electromagnetic fields could also disturb fish migration patterns by interfering with their capacity to orientate themselves in relation to Earth’s magnetic field (Bergström et al. 2014). However, the production of these electromagnetic fields can be mitigated by proper cable design (Bergström et al. 2014). The major concerns for this phase of offshore wind farms are seabird mortality caused by collision with the moving turbine blades and seabird displacement from key habitats as a result of avoidance responses (Bailey et al. 2014). These issues can affect birds migrating through the area as well as those that breed or forage in the vicinity.
Although the operation of offshore windmills has negative impacts on the local ecosystem, these impacts may be matched or even outweighed by the positive ones that wind farms also introduce. Windmills can produce habitat gain by acting as artificial reefs, thereby enhancing local species abundances and biodiversity (Bergström et al. 2014). Moreover, studies have found that fish are seasonally attracted to wind farms with high fidelity, and that seals are potentially using wind farms as foraging sites (Reubens et al. 2014), (Russell et al. 2014). However, increased species abundance can be problematic when some species benefit more than others, or when wind farms introduce non-indigenous species (Bergström et al. 2014). Another major positive contribution wind farms make to the local ecosystem is fisheries exclusion. Although wind farms restrict fisheries’ intrusions for the safety and preservation of the farm itself, the local species benefit, both targeted species and non-targeted bycatch species (Bergström et al. 2014). This exclusion also prevents bottom trawling, the dragging of nets on the sea floor, so benthic organisms benefit as well (Bergström et al. 2014). Moreover, an abundance of fish caused by the exclusion of fisheries may have spillover, and surrounding areas may also see an increase in species abundance (Bergström et al. 2014). There may also be opportunities in the future to combine offshore wind farms with open ocean aquaculture (Bailey et al. 2014). With notable contributions to biodiversity and species abundance, windmill farms mitigate their negative impacts on the local environment.
With an increasing demand for renewable energy and, by extension, wind energy, offshore wind farms will continue to develop and grow around the world. Therefore, an increasing understanding of the impacts offshore wind farms have on marine environments is needed. Currently, investigations show that the construction phase consistently inflicts negative impacts, and that pressures during the operational phase may impose both negative and positive effects, depending on local environmental conditions as well as prevailing management targets (Bergström et al. 2014). However, confidence in these findings is not perfect because of their variable nature and because of the relatively short time frame offshore wind farms have operated, limiting data collection. In order to gain a better understanding of potential impacts, conservationists need to further investigate population dynamics and migrations in areas that already have wind farms and areas that are under consideration for them.
References:
IEA (2013), World Energy Outlook 2013, IEA. doi: 10.1787/weo-2013-en
Bergström, L., Kautsky, L., Malm, T., Rosenburg, R., Walberg, M., Capetilli, N. Å., and Wilelmsson, D. (2014) Effects of Offshore Wind Farms on Marine Wildlife – A Generalized Impact Assessment. Environmental Research Letters, 9. doi:10.1088/1748-9326/9/3/034012
Bailey, H., Brookes, K.L., and Thompson, P.M. (2014) Assessing Environmental Impacts of Offshore Wind Farms: Lessons Learned and recommendations for the Future. Aquatic Biosystems, 10:8. doi:10.1186/2046-9063-10-8
Reubens, J.T., Degraer, S., and Vincx, M. (2014) The Ecology of Benthopelagic Fishes at Offshore Wind Farms: A Synthesis of 4 Years of Research. Hydrobiologia, 727: 121-136. doi:10.1007/s10750-013-1793-1
Russell, D.J.F., Brasseur, S.M.J.M., Thompson, D., Hastie, G.D., Janik, V.M., Aarts, G., McClintock, B.T., Matthiopoulous, J., Moss, S.E.W., and McConnell, B. (2014) Marine Mammals Trace Anthropogenic Structures at Sea. Current Biology, 24(14): R638- R639. doi:10.1016/j.cub.2014.06.033